How to Prepare Human Pancreatic Cancer Organoids

Pancreatic cancer is one of the most common malignant tumors of the digestive system and is often referred to as the "king of cancers" in the field of oncology. According to The Lancet, the five-year survival rate for pancreatic cancer is approximately 10%, making it one of the cancers with the worst prognosis. Clinical symptoms of pancreatic cancer are often obscure and atypical, making both diagnosis and treatment challenging for this malignancy of the digestive system. Therefore, organoid models suitable for studying the physiological characteristics and treatment options of pancreatic cancer are crucial as disease models and in regenerative medicine in the field of pancreatic research, attracting increasing attention and usage by researchers.
Current Status of Pancreatic Organoids
Currently, organoid culture techniques have made significant progress in many organs such as the stomach, intestine, liver, bile duct, and brain. The pancreas is the only organ in the human body that serves as both an exocrine and endocrine gland, posing significant challenges to the development of pancreatic organoid technology.
The pancreas is divided into an exocrine part and an endocrine part. The exocrine gland consists of acini and ducts, while the endocrine gland consists of islets of Langerhans. The exocrine gland is the origin of pancreatitis and pancreatic ductal adenocarcinoma (PDAC). Our understanding of the origin and progression of human pancreatic diseases, including PDAC, is limited. Although techniques for pancreatic ductal and islet organoids are improving, maintaining acinar and ductal cells in vitro remains challenging. Therefore, constructing complex pancreatic organoids remains a current research bottleneck.
Pancreatic organoids can be derived from healthy pancreas or pancreatic tumors and serve as an important translational bridge between in vitro and in vivo models. The induction of pancreatic ductal and acinar organoids from human pluripotent stem cells has been reported, and these organoids exhibit characteristics of neonatal exocrine pancreas. We have developed a renewable source of ductal and acinar organoids for simulating exocrine development and diseases. The specific protocol and procedures are as follows:
Culture Protocol
1. Cell Preparation
Pancreatic progenitor (PP) cells were extracted from Hues-8 cells by the research team, collected at the S1+4 d induction stage, and dissociated into single cells using TrypLE. The digested cells were centrifuged at 1500 rpm for 5 minutes, then resuspended in differentiation medium containing 5% Matrigel, resulting in a final cell density of 50,000 cells/mL. The cell suspension was seeded onto culture plates precoated with 100% Matrigel, and the medium was changed every four days with supplementation of 5% Matrigel.
2. Pancreatic Ductal Induction
For ductal cell induction, differentiation (alkaline) medium consisted of DMEM, 1% Pen-Strep, 1% B-27, 10 ng/mL FGF-1, 50 μg/mL ascorbic acid, 1 μM A 83-01, 10 ng/mL FGF-10, and 1 ng/mL EGF.
Phase I (Days 0-4): Ductal induction medium contained alkaline medium supplemented with 10 μM Y-27632, 50 nM SB939, 3 μM IWP-2, 25 nM IQ-1, 50 nM iCRT14, 15 ng FGF-10, 10 ng/mL FGF-2, and 5 ng/mL EGF.
Phase II (Days 4-8): Ductal induction medium contained alkaline medium supplemented with 3 μM IWP-2, 25 nM IQ-1, 50 nM iCRT14, and 5 μM Y-27632.
Phase III (Days 8-12): Ductal induction medium contained alkaline medium supplemented with 3 μM IWP-2, 25 nM IQ-1, 50 nM iCRT14, and 0.1 μM XAV-939.
Phase IV (Day 12): Ductal induction medium contained alkaline medium supplemented with 3 μM IWP-2, 5 nM IQ-1, and 10 nM iCRT14.
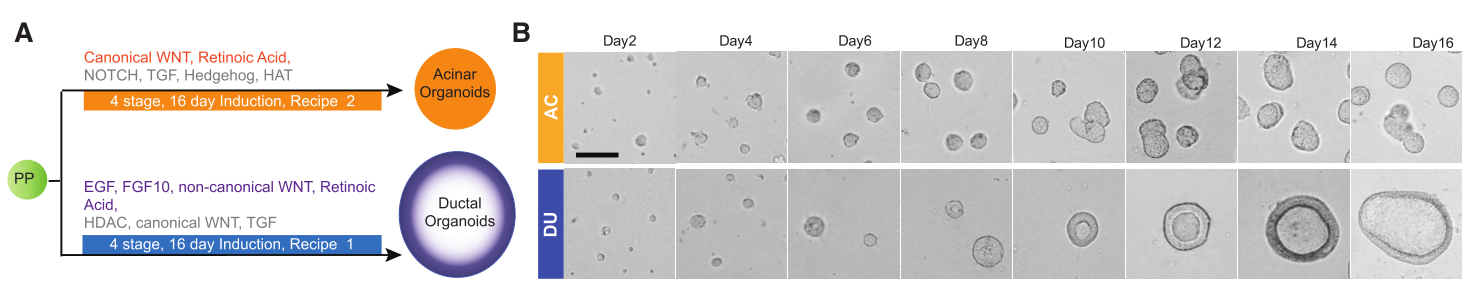
Figure 1.A. Schematic of Ductal-like and Acinar-like Organoid Induction Protocols.B. Phase-Contrast Images of Organoids at Day 16 of Culture (n = 3, independent cultures), Scale bar, 50 millimeters.
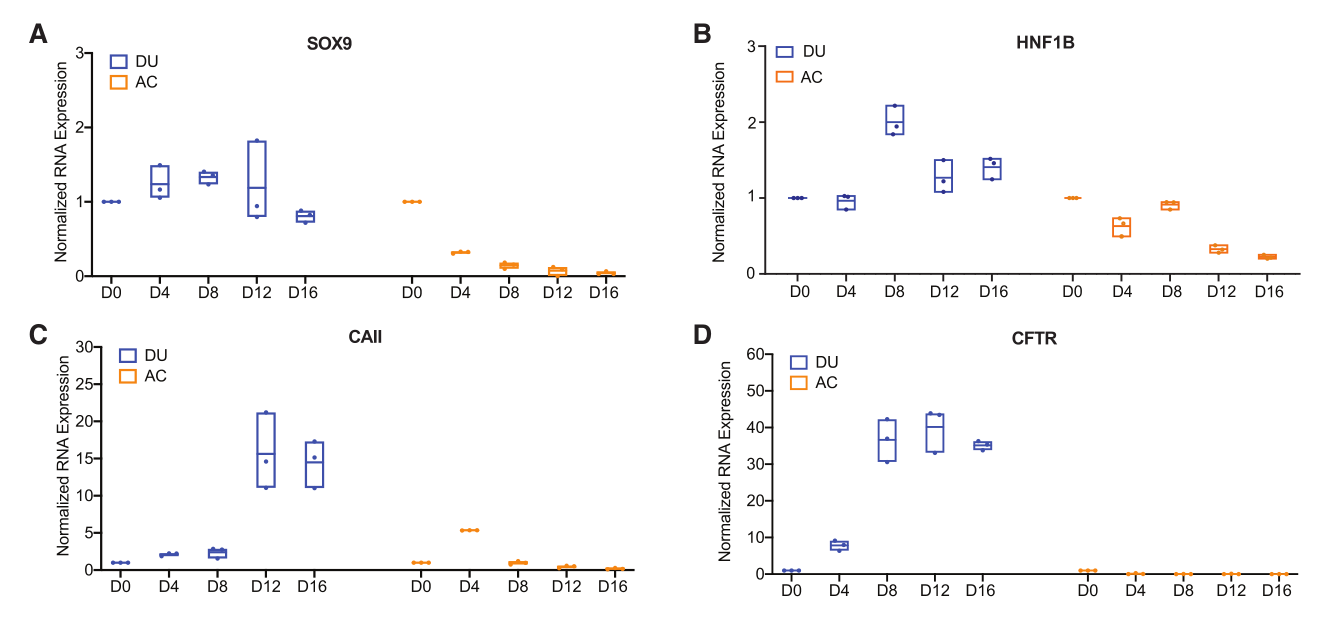
Figure 2. RNA Expression of Pancreatic Ductal Markers SOX9 (A), HNF1B (B), CAII (C), and CFTR (D) in DU and AC Cultures after 16 Days of Culture.
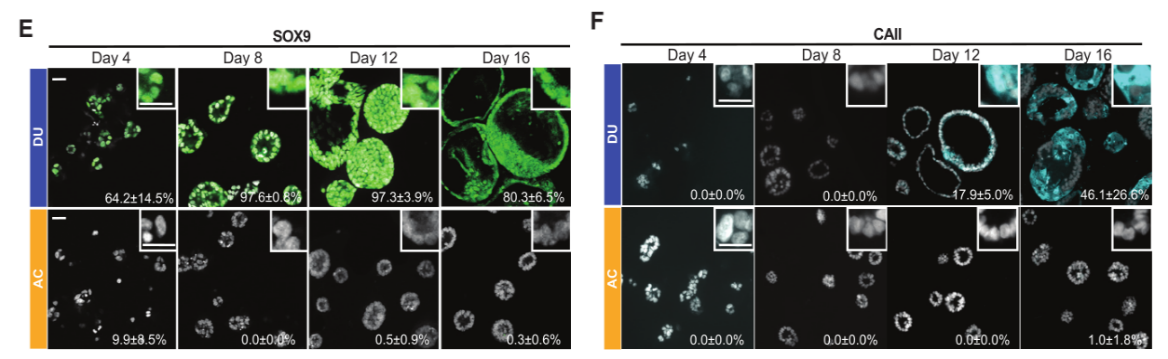
Figure 3. Immunofluorescence Detection of SOX9 (E) and CAII (F) Expression.
3. Pancreatic Acinar Induction
For acinar induction, the alkaline culture medium contains DMEM, 1% Pen-Strep, 1% B-27, 10 ng/mL FGF-1, 50 μg/mL ascorbic acid, 1 μM A 83-01, and 10 ng/mL WNT1.
I Phase Acinar (Day 0-4) culture medium consists of the alkaline culture medium supplemented with 10 μM Y-27632, 3 μM SKL 2001, 50 nM dexamethasone, 0.3 μM CHIR-99021, 50 nM XMU-MP-1, 15 ng/mL WNT1, 5 ng/mL FGF-2, 1 ng/mL EGF, and 5 ng/mL FGF-10.
II Phase (Day 4-8) acinar culture medium contains the alkaline culture medium supplemented with 3 μM SKL 2001, 200 nM dexamethasone, 0.1 μM LDN193189, and 50 nM XMU-MP-1.
III Phase (Day 8-12) acinar culture medium contains the alkaline culture medium supplemented with 3 μM SKL 2001, 200 nM dexamethasone, 0.1 μM LDN193189, and 1 μM DBZ.
IV Phase (Day 12) acinar culture medium contains the alkaline culture medium supplemented with 0.3 μM SKL 2001, 25 nM dexamethasone, and 0.1 μM DBZ.
For experiments involving TGF-β treatment, A 83-01 is removed from the culture medium starting from Day 83.
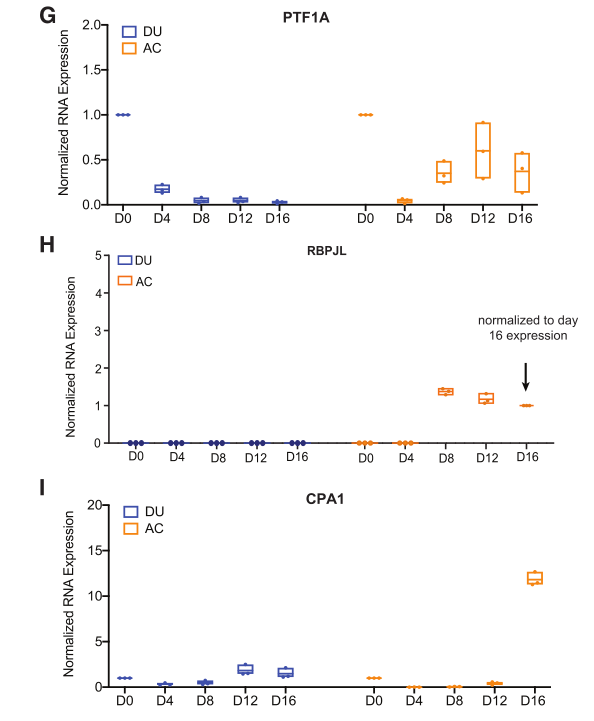
Figure 4. RNA expression of pancreatic acinar markers PTF1A (G), RBPJL (H), and CPA1 (I) in DUs and acinar cultures after 16 days of cultivation.
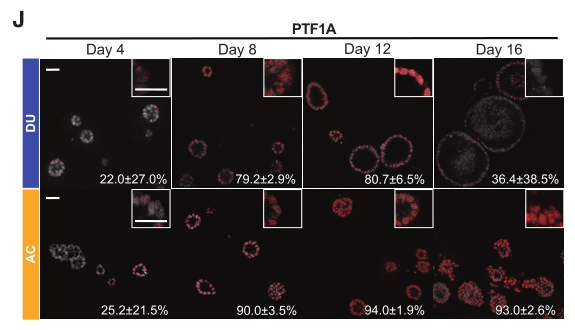
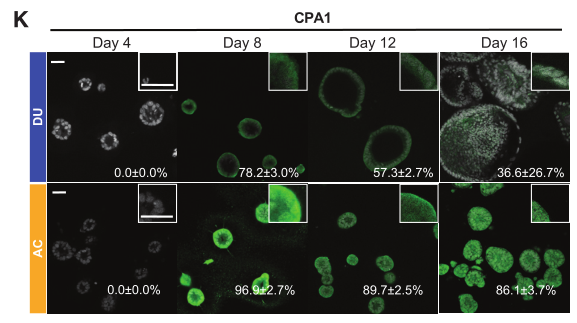
Figure 5. Immunofluorescence detection of PTF1A (J) and CPA1 (K) expression in organoids.
Compound Information Summary as Follows
|
Product Name |
Catalog Number |
Specification |
| 3DCultr Pancreatic cancer Organoid Growth Medium (Human) | C231113 | 50/100/500 mL |
|
Recombinant Human Acidic Fibroblast Growth Factor (Human aFGF) |
C230286 |
10/100/500 μg |
|
Recombinant Human FGF-10 Protein, His Tag |
C230418 |
5/100/500 μg |
|
Recombinant Human bFGF/FGF-2 Protein |
C230295 |
10/100/500 μg |
|
Recombinant Human EGF Protein |
C230328 |
100/500 μg |
