Common Issues and Solutions in Annexin-V Method for Detecting Cell Apoptosis

The Annexin V-FITC/PI Cell Apoptosis Detection Kit utilizes FITC-labeled Annexin V as a probe to detect the occurrence of early apoptosis in cells, making it the most commonly used probe combination in cell apoptosis detection experiments.
Detection Principle
During the early stages of cell apoptosis, phosphatidylserine (PS) on the inner side of the cell membrane is translocated from the cytoplasm to the extracellular space, exposing itself to the external environment, and increasing the permeability of the cell membrane, thereby being labeled by the PS-specific Annexin-V probe.
Annexin-V is a calcium-dependent phospholipid-binding protein with strong affinity for PS. PI is an economical and stable nucleic acid dye that cannot penetrate intact cell membranes of normal or early apoptotic cells, but can penetrate the cell membranes of late apoptotic and necrotic cells, staining the cell nuclei red.
When Annexin V is used in conjunction with PI, PI is excluded from live cells and early apoptotic cells, while late apoptotic cells and necrotic cells are simultaneously stained double-positive with FITC and PI.
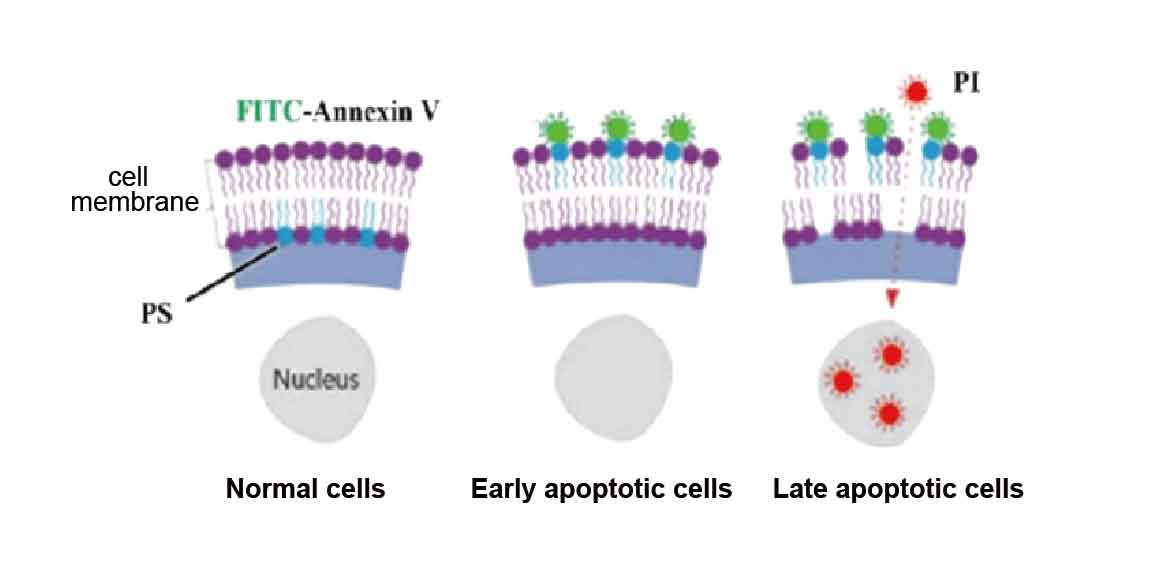
Figure 1. Principle of Annexin V-FITC/PI Cell Apoptosis Detection
Flow Cytometry Analysis
FITC has a maximum excitation wavelength of 488 nm and a maximum emission wavelength of 525 nm. The green fluorescence of FITC is detected in the FL1 channel.
The PI-DNA complex has a maximum excitation wavelength of 535 nm and a maximum emission wavelength of 615 nm. The red fluorescence of PI is detected in the FL2 or FL3 channel.
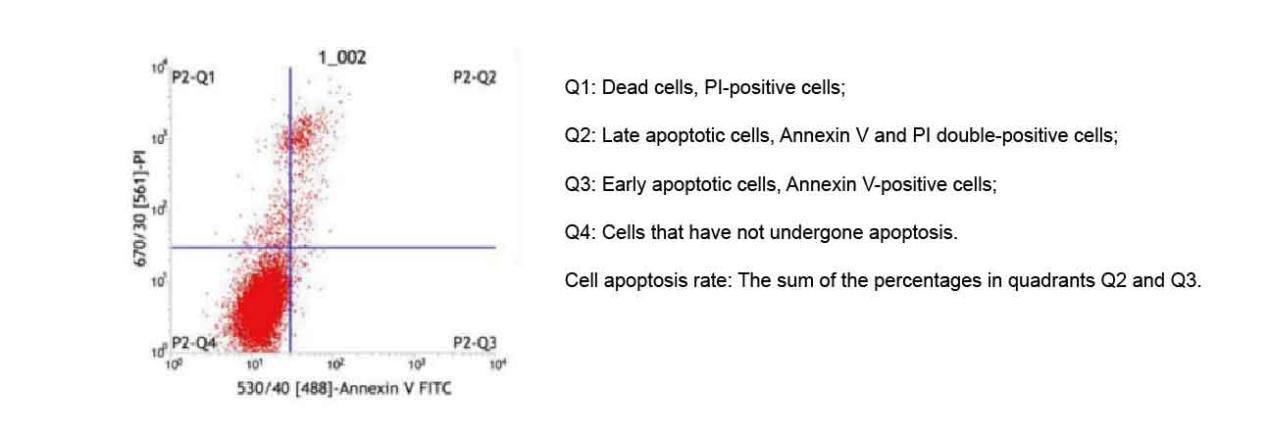
Figure 2. Annexin V-FITC/PI Cell Apoptosis Detection Results
During apoptosis experiments, false positives or results that do not match expectations often occur. Possible reasons include improper compensation adjustment, excessively high cell concentration, over-digestion, drug interference, excessive drug treatment time, sample self-issues, among other factors. Next, I will show you how to obtain a perfect result image.
01. Improper Compensation Adjustment: Fluorochromes emit fluorescence at different wavelengths when excited by lasers. In theory, each fluorescence can be detected by the appropriate detector through the selection of suitable filters, without interference from other fluorochromes. However, these fluorochromes currently have broad emission spectra. Although their emission peaks are different, there is some overlap in the emission spectra range. In other words, adjacent detectors will detect fluorescence signals from each other, affecting the accuracy of the detection results. Therefore, spectral overlap correction must be performed during multicolor analysis.
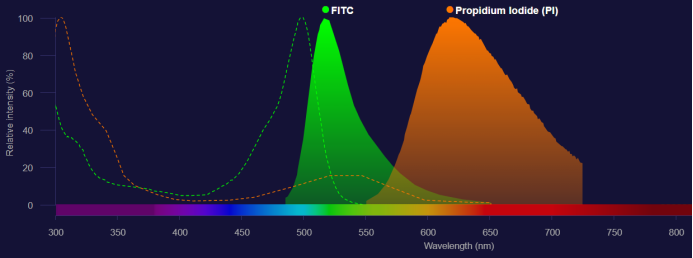
Figure 3. Emission Spectra of FITC and PI
Adjustment Method: Setting Controls
Blank Tube: Cells induced to undergo apoptosis without dye are used to adjust the photomultiplier tube voltage for fluorescence channels.
Single-Stained Tubes: Tubes containing only PI and Annexin V labeled with fluorescent markers are separately needed to adjust the compensation values for the two fluorescence channels. The compensation values between fluorochromes should remain constant. During dual fluorescence staining, if one fluorescence is strong and the other is weak, compensation must be performed. Compensation should only be applied between different fluorochromes excited by the same laser to avoid undercompensation and overcompensation. [3]
02. Excessive Cell Concentration: Excessive cell concentration leads to rapid depletion of the culture medium, causing starvation-induced apoptosis. Therefore, efforts should be made to ensure that cells are in optimal condition before inducing apoptosis in cells. Subsequently, apoptosis induction treatment should be performed to avoid apoptosis caused by cellular senescence.
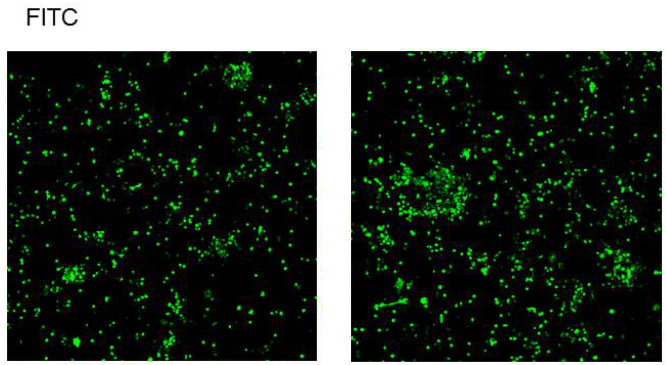
Figure 4. Excessive Cell Concentration Leading to Spontaneous Apoptosis, Blank Control (Left), Experimental Group (Right)
03. Excessive Cell Digestion: For adherent cells, digestion is a critical step, and the digestion solution should contain trypsin without EDTA because the binding of Annexin V and PS requires Ca2+, while EDTA is a chelating agent for Ca2+. The use of trypsin containing EDTA can result in false negatives. During cell digestion, trypsin can be spread over the bottom of the well plate and gently shaken to ensure thorough contact with the cells. Then, most of the trypsin should be removed, and the remaining small amount of trypsin can be used for further digestion. When the gaps between cells increase and the bottom of the bottle becomes spotted, digestion can be terminated. Gentle pipetting is necessary to avoid excessive digestion and mechanical damage, both of which can cause damage to the cell membrane and result in false positives.
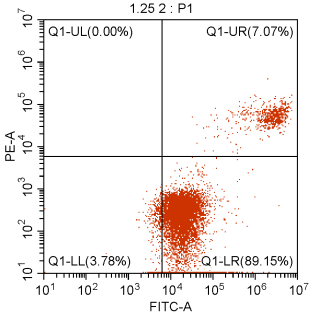
Figure 5. Excessive Cell Digestion Leading to 89.15% Early Apoptotic Cells
04. Fluorescence Interference from Apoptosis-Inducing Drugs: In the image below, the untreated blank control group (left) shows early apoptotic cells accounting for 3.38% and late apoptotic cells accounting for 6.64%. However, in the image on the right, cells in quadrants Q1 and Q2 account for 90%, with nearly all cells producing fluorescence in the PI channel, indicating fluorescence interference from the drug. Therefore, when selecting a reagent kit, one should try to avoid interference from drugs and endogenous fluorescence signals from cells.
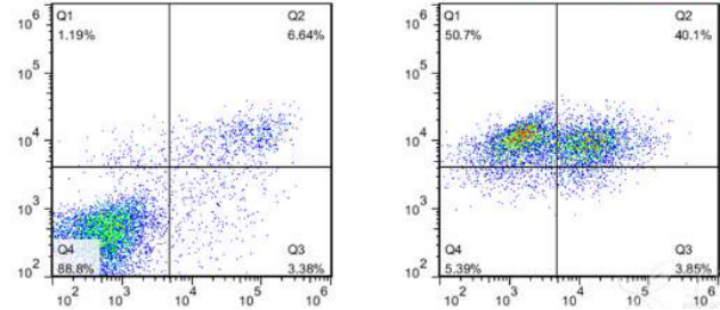
Figure 6. Fluorescence Interference from Drug Itself: Blank Control (Left) and Cells Treated with Drug (Right)
05. Prolonged Sample Storage or Excessive Drug Treatment Time: After sample processing, flow cytometric analysis should be conducted promptly. Prolonged sample storage or excessive drug treatment time can also lead to cells entering the late apoptotic and necrotic stages. As shown in the image below, the blank control group shows normal results, but the drug-treated group exhibits a large number of late apoptotic cells, indicating that the prolonged drug treatment time may be the cause.
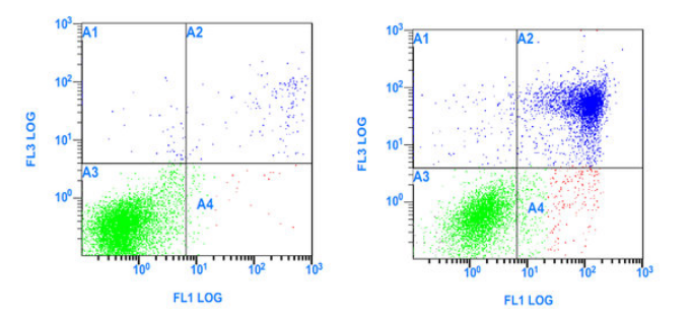
Figure 7. Excessive Drug Treatment Time: Blank Control (Left) and Cells Treated with Drug (Right)
06. Detection Sample: Neuronal Cells
(1) If the detection sample consists of neuronal cells, the neuronal cell membrane is prone to damage and flipping, leading to false positives. Therefore, the Annexin V/PI double staining flow cytometry method for detecting cell apoptosis is not suitable for neuronal cells.
(2) Detection Sample Source: Blood
If the sample is derived from blood, it is essential to remove platelets from the blood. Platelets contain PS, which can bind to Annexin V and interfere with the experimental results. To remove platelets, a buffer containing EDTA can be used, followed by centrifugation at 200 g.
